Supercritical fluids
Supercritical fluids (SCFs) are substances above their critical point of temperature and pressure. In general terms, SCFs have properties between those of a gas and a liquid. By changing the pressure and temperature of the fluid, the properties can be tuned to be more liquid-like or more gas-like. SCFs can be viewed as a hybrid of liquid and gases that offer tunable solvent properties and unique advantages for the study and processing of nanoscale materials.
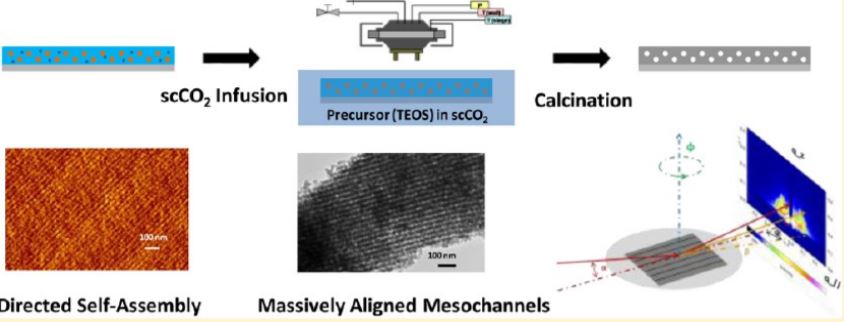
In the group we have experience using SCFs as a tool to prepare hierarchical materials, including mesoporous metal oxides and carbons, by the three-dimensional replication of block copolymer templates in supercritical carbon dioxide, or the reactive deposition of metals with high aspect ratio features and the conformal deposition of metal oxide films from a CO2 solution.
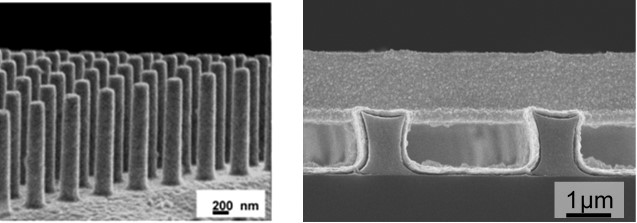
Figure. SEM micrograph of self-supporting gold arrays (left), and conformal deposition of a ruthenium metallic film (right).
Related publications:
- Haruki, M., Li, S., Qian, G. and Watkins, J.J., 2016. Reactive deposition of cobalt using bis (2, 2, 6, 6-tetramethyl-3, 5-heptanedionato) cobalt (II) from supercritical carbon dioxide. The Journal of Supercritical Fluids, 107, pp.189-195.
- Zong, Y. and Watkins, J.J., 2005. Deposition of copper by the H2-assisted reduction of Cu (tmod) 2 in supercritical carbon dioxide: kinetics and reaction mechanism. Chemistry of Materials, 17(3), pp.560-565.
- Watkins, J.J. and McCarthy, T.J., 1995. Polymer/metal nanocomposite synthesis in supercritical CO2. Chemistry of Materials, 7(11), pp.1991-1994.